Working Time
| Mon-Sat | 09:00 - 5:00 |
| Sunday | Closed |
25 YEARS OF RESEARCH AND DEVELOPMENT
VARIED DESIGNS TO SUITS DIFFERENT ANATOMIES
LONG TERM WELL DOCUMENTED CLINICAL RESULTS
SURGEON FRIENDLY INSTRUMENTATION
FDA APPROVED
ISO: 13485 - 2016
ISO: 9001 - 2015
CLEAN ROOM MAINTAINED AS PER STANDARDS CLASS 100,000/ISO8
UNIQUE FEATURES
» Technically Strong, Professional Organization
» Clean Room Maintained As Per Standards Class 100,000/ISO8.
» CNC Machines And Latest Equipments To Maintain Quality and Quantity of Implants And Instruments.
» Well Documented, Followed And Maintained Procedures.
» Stringent Quality Check Procedures At All Levels Of Manufacturing.
» 3rd Party Quality Check Of Raw Materials And Casting.
» Emphasis on Research And Development For All Processes - From Designing To Manufacturing.
» Accredited with FDA, ISO : 13485 : 2016 & ISO 9001 : 2015.
ENHANCED STABILITY.
PROVEN MODULARITY.

CORPORATE VIDEO
E TABLE COFFEE BOOK ON MEDICAL DEVICES
3DGEM 2023
LEADERS OF TOMORROW AWARDS SEASON 11
OUR PRODUCTS
BIPOLAR HIP PROSTHESIS
Bipolar prostheses is a monoblock design where the hip movement occurs between the prosthesis and the acetabulum (hip socket). A bipolar prosthesis has an additional artificial joint between the two components of the prosthesis. Both treatments are clinically proven and common around the world.


MODULAR GEN X HIP PROSTHESIS
It's triple tapered and polished stem designed to be compatible with cement shroud. Centralizers are available for all the stem sizes for placing the stem in the centre of femoral canal.
MODULAR MULLER HIP PROSTHESIS
Muller is a complete solution for total hip arthroplasty. The design gives primary stability and is a favourable solution for young and old patients. Based on the design of M.E. Muller straight stem, the proximal chamfer on the stem is very MIS friendly. We have a surgeon friendly instrumentation for Muller Hip Prosthesis.
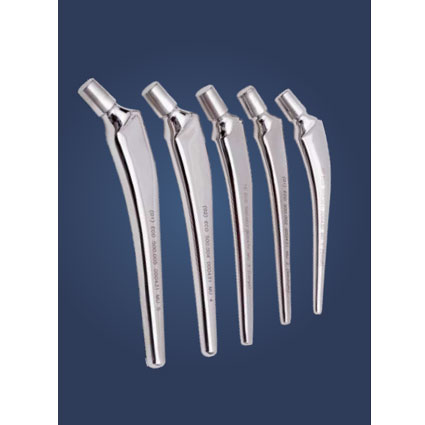

RING LOCK MODULAR HEAD
The ring lock modular head implants provide an additional load bearing surface compared to a traditional implant. It prevents dislocation and assist instability, longevity It advance fixation for fractures in primary or revision total hip arthroplasty cases.
MODULAR FEMORAL HEAD
Modular femoral neck designs can make a complex operation easier by supplying more options and offering more flexibility. Modular femoral head adds to the modularity as it allows to adjust offset, version and limb length and to make adjustments to these parameters independently of one another during preoperative planning.

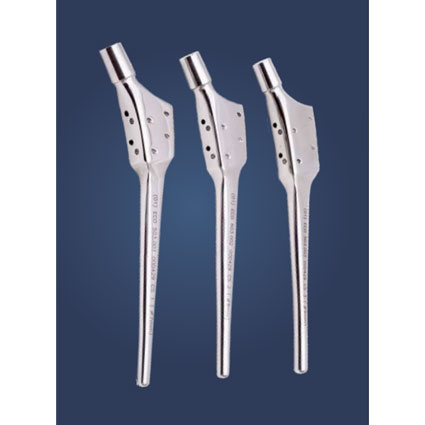
MODULAR CALCAR HIP PROSTHESIS
Use in case of collapse of the neck structure or loss of bone stock. Bone deficiencies in the proximal/ medial portion of the femur. The stem can be individually positioned to reconstruct femoroacetabular offset and caput-collum-diaphyseal angle in varus or valgus hips, thereby achieving soft tissue balance around the joint.
CEMENTED ACETABULAR CAGE
The rationale for the use of acetabular cage is to provide mechanical stability to the acetabular construct, protect bone graft/cup/augments transmitting the load to the host bone through the cage. The advantage of an acetabular component in a correct anatomic level is to restore hip biomechanics and stability more accurately.

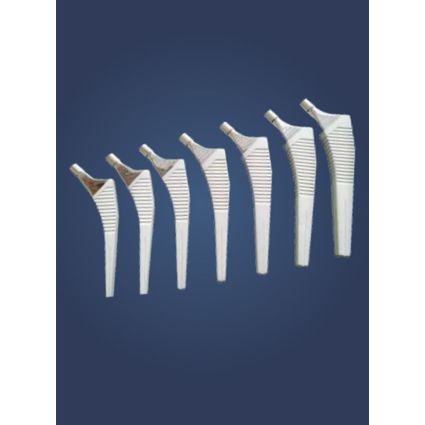
UNCEMENTED PLATONIC HIP PROSTHESIS
Platonic is a complete solution for total hip arthroplasty. The design gives primary stability and is a favourable solution for young and old patients. The HA coating offers excellent secondary stability and the accurate instruments ensure an easy implantation. We have a surgeon friendly instrumentation for Platonic Hip Prosthesis.
UNCEMENTED LONG HIP PROSTHESIS
A femoral hip implant is required as a long distal section of the femoral component is used for revision surgery or in case of a fracture in the femur or a bone defect. A long stem component is essential if a major defect exists in the cortex of the proximal portion of the femur.

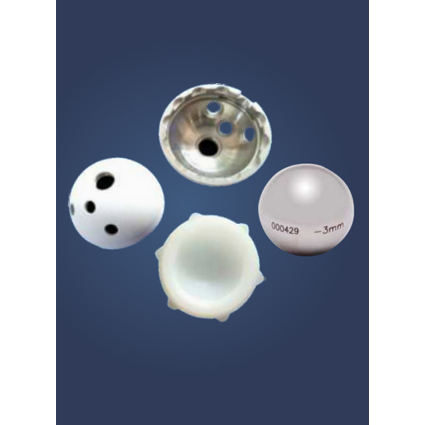
UNCEMENTED CUP, SHELL & LINER
A versatile modular design is a revolutionary technology engineered for rapid bone in growth, strong, flexible yet versatile to give maximum motion. The liner strengthens the locking system between the cup It the shell. It has multiple augment options It designed to help maximum acetabular stability, thus preventing dislocation.
DR. MUKHERJI'S SHOULDER PROSTHESIS
There are 2 types of shoulder replacement surgeries.
1. Monoblock Shoulder Replacement
2. Modular Shoulder Replacement
Also known as shoulder arthroplasty. It is, the removal of portions of the shoulder joint, which are replaced with artificial implant to reduce pain and restore range of rotation and mobility.


ELBOW PROSTHESIS
A total arthroplasty (Elbow replacement) is a surgical procedure in which parts of an arthritic joint are replaced with a prosthesis. One part fits into the upper arm (humerus) and the other part fits into the forearm (ulnar). The two parts are then connected and held together by a pin. The primary function of the elbow joint is to bend and straighten the arm.

